|
|
|
|
|
|
|

|
| MTM Newsletter |
N° 18 - September 2016 | |
|
|
|
| |
|
|
| |
|
|
| |
|
|
|
Laboratory News
|
 How valid are hair mercury levels? How valid are hair mercury levels?
|
An Australian Naturopath asked this question and we answered:
Just recently, we completed at Quality Assessment Scheme of hair,
urine and blood samples with the Quebec Centre of Toxicology. Our test
results were within the given ranges for all elements with the exception
of Thorium and Tin in blood. For these elements, our blood results were
marginally below the low range, hair and urine values were within the
expected norm. Our mercury results for hair, blood or urine were
excellent.
The analysis of mercury in hair is not a problem in our laboratory
and I can explain why many therapists still think mercury in hair is
not relevant.
To do elemental testing in hair, we need to break down metal
complexes before the analysis can take place. In the old days, we added
metal-free acids to the sample tube, which remained open during the digestion period using heat. During this process, sulfur fumes and highly volatile elements such as Mercury escaped.
Today, we digest samples in closed tubes in microwave ovens.
Nothing escapes. Under those conditions, mercury results reliably
reflect the mercury concentration in hair.
As long as a metal circulates in blood, it can be deposited in tissue. If nothing circulates, nothing can be stored.
Toxins such as mercury have a great tendency to 'hide' in fatty
tissue and are difficult to be accessed. They are basically fixed in
place and are not released into the blood stream. Chelation is generally
ineffective, unless we allow the body to redistribute. This takes time,
meaning treatment pauses are essential. During weight loss, we also
release toxins.
|
 Detoxifying
Uranium (U) Detoxifying
Uranium (U)
|
Our report, Uranium and other contaminants in hair from the parents of children with congenital anomalies in Fallujah, Iraq,
published in Conflict and Health 2011, drew attention to the
possibility that depleted uranium is the cause of the increase in
congenital birth anomalies and cancer in Fallujah, Iraq. We determined
high levels of uranium and other potentially toxic metals in the hair of
the Iraqi test groups, and have since found similar patterns in Indian
children with physical and mental disabilities. We frequently find
elevated uranium in hair and urine of people without disabilities and as
a result, the question on how to remove uranium is asked.
The ATSDR (Agency for Toxic Substances and Disease Registry) lists key points:
- Everyone is exposed to uranium in food, air, and water as
part of his/her natural environment. Most exposures do not warrant
monitoring or treatment.
- Strategies for treatment and management of overexposed
patients include removal from overexposure, decontamination, monitoring
uranium biomarkers of exposure and biomarkers of effect
(nephrotoxicity), administration of sodium bicarbonate to maintain an alkaline urine, and pushing fluids to increase urine output.
- Nephrotoxicity should reverse as overexposure ceases.
On our website, we recently focused on news from the researchers
at the Helmholtz-Center Dresden-Rossendorf (HZDR), Germany and the
University of Bern, Switzerland. Their work focused on the effect of
uranium toxicity on microorganism and plant life. They reported that
Glutathione efficiently decreases the chemical toxicity of uranium,
meaning microorganism show a greater tolerance for uranium. Karim Fahmy
explained, "We noticed that uranium binds to the carboxyl groups of
glutathione, which results in an insoluble and nontoxic complex."
Reference:
Muhammad H. Obeid, Jana Oertel, Marc Solioz, Karim Fahmy,
Mechanism of attenuation of uranyl toxicity by glutathione in
Lactococcus lactis, in: Applied and Environmental Microbiology June 2016
The following graph indicates that only the mean values of
uranium in baseline urine (0.069mcg/g creatinine) slightly exceed the upper
norm of 0.60mcg/g creatinine, meaning slight exposures are noted. Our
statistical evaluation of various urine provocation test indicates that
none of the chelating agents, used alone or in combination with other
chelation agents, shows promise. This is in accordance with information
provided by Poison Center and various governmental agencies.
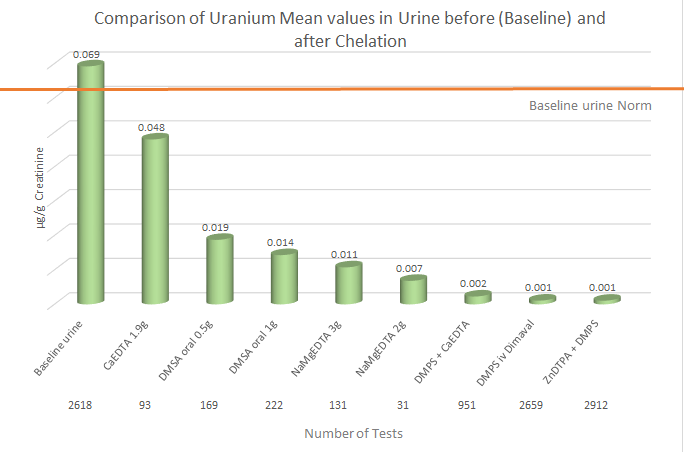
Source: Micro Trace Minerals Database 2016
Summary: Alkalizing the urine and paying attention to fluid
intake and output seems the most appropriate way to treat a uranium
exposure.
|
 Gadolinium (GD) Gadolinium (GD)
|
The contrasting agent Gd-DTPA (Gadopentetat-Dimeglumin) is used for
MRIs. As the name indicates, the element Gd is complexed with the
chelating agent DTPA (Diethylentriamine penta acetate). Unless
complexed, gadolinium is toxic to humans. The pharmacokinetic behavior
of intravenously delivered Gd-DTPA is similar to the well-known
iodinated contrast agents used in urography and angiography; excretion
of the Gd-DTPA complex is predominantly through the kidneys with greater
than 90% recovery in 24 hour.
The element gadolinium is used in industry for the manufacture of
electronic components, magnets and superconductors used for nuclear,
microwave and radar technology.
Our data indicates that even days after an MRI, Gd-values are
extremely high in unchallenged baseline urine, but decrease over time.
At this time, we do not know how long traces of Gd remain in the system.
When we analyze for Gd, we break down the metal complex and report
the element Gd only. Our data base indicates that provocation urines may
show extreme values as well, but unless we compare baseline values with
provocation test results, we do not know if the Gd concentration found
in a provocation urine is the result of the chelation therapy or simply
reflects the baseline value.
|
 Sampling Instructions - We are asking for your help Sampling Instructions - We are asking for your help
|
If sampling is done outside your office,
please inform your patient of the following:
-
Do not fill tubes up to the rim, (see picture) esp. urine and stool samples.
It can make for an awful mess.
-
Label tubes! It prevents sample mix-ups.
-
Provide sufficient information by filling out the patient information sheet.
The information helps us with quality and probability controls.
-
Please inform patients of laboratory costs,
which are listed on our Patient Information Sheet.
|
-
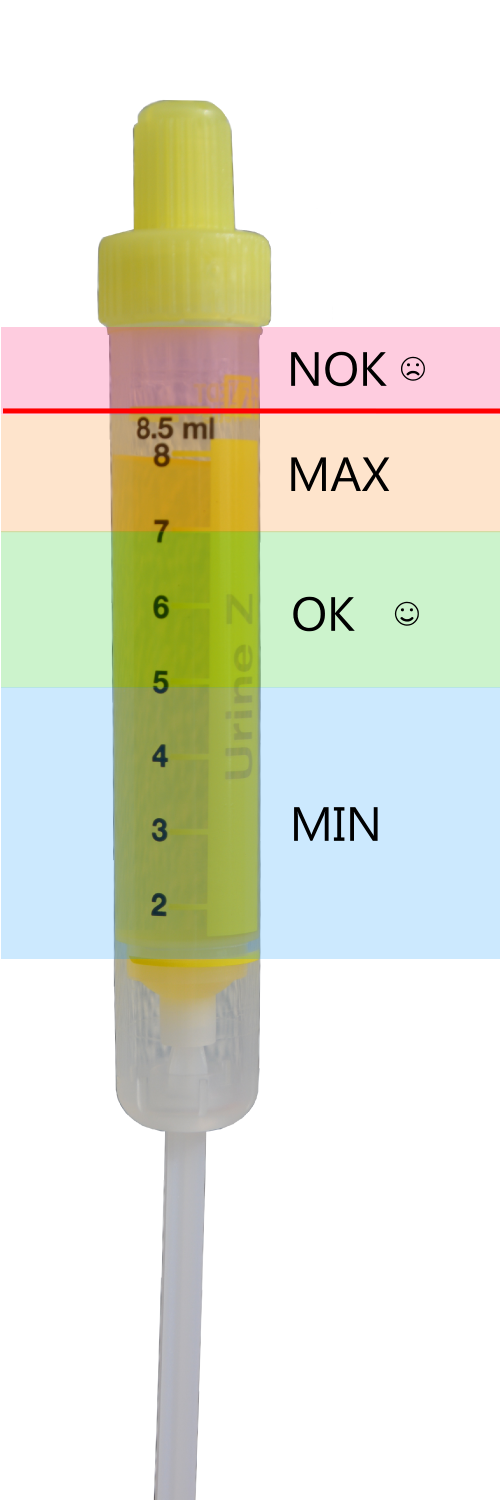
Tube filling marks
|
|
 Aluminum and Barium in CaEDTA ampules Aluminum and Barium in CaEDTA ampules
|
In our previous newsletter we focused on Aluminum. The chelator
Desferal can be used for aluminum overexposure after the diagnoses has
been verified in blood, baseline urine, stool and other diagnostic
means, but administration of this drug can cause considerable side
effects. We therefore evaluated the aluminum binding of the more common
chelating agents and noticed an unusual percentage of slightly elevated
aluminum values in NaCaEDTA provocation tests. This seemed to indicate
that CaEDTA is a chelator of choice for aluminum or most people
receiving this chelator are overexposed to aluminum.
Consequently, we tested one ampule of NaCaEDTA and found over
1200mcg/l Al and about 1900mcg/l Barium. This means, this 5ml ampule
contain about 6mcg Aluminum and nearly 10mcg of Barium. No aluminum or
barium could be detected in Dimaval ampules (DMPS from Heyl, Berlin). We
also tested the raw material NaEDTA and found negative Al and Ba
results.
On a note: We routinely test chelating agents either at doctors
requests or because our routine statistics point towards potential
problems. We always inform the manufacturer accordingly.
|
| |
| |
|
|
| |
|
|
|
Medical Workshops and Conferences
|
 International Conferences & Workshops 2016 International Conferences & Workshops 2016
|
| 09/24/2016 |
Chelation Therapy Workshop
Novotel Hamburg City Alster, Luebeckerstr. 3
Hamburg, Germany (German) |
| 10/30/2016 |
Presentation by Dr. E. Blaurock-Busch
Risk reduction with Chelation Therapy as Interval Therapy in Cancer Patients
Congress Center Baden-Baden
Baden-Baden, Germany (German / English)
For more information, please visit:
http://www.medwoche.de/programm/english-programme-2016.html
|
|
 Webinar Webinar
|
| 10/12/2016 |
Use of chelating agents (DMPS, DMSA, EDTA, DTPA) and diagnostic tests to confirm treatment success.
Online Seminar registration here:
https://www.edudip.com/w/207192
|
|
For future workshops and updates, please visit:
https://microtraceminerals.com/en/workshops
|
|
| |
| |
|
|
|
|
|
|
|
If you have any questions please feel free to contact us.
E. Blaurock-Busch and Team
|
|
|
|
| |
|
|
| |
|
|
|
